46+ The Most Complete Amphoteric Oxides In Periodic Table. The periodic table consists of seven periods, from period 1 to period 7. Amphoteric oxides react with both acids and bases (i.e. Sno2 is an amphoteric oxide because it reacts with acids as well as bases to form corresponding salts.
46+ The Most Complete Amphoteric Oxides In Periodic Table The following sciencestruck article will cover some information related to metalloids.
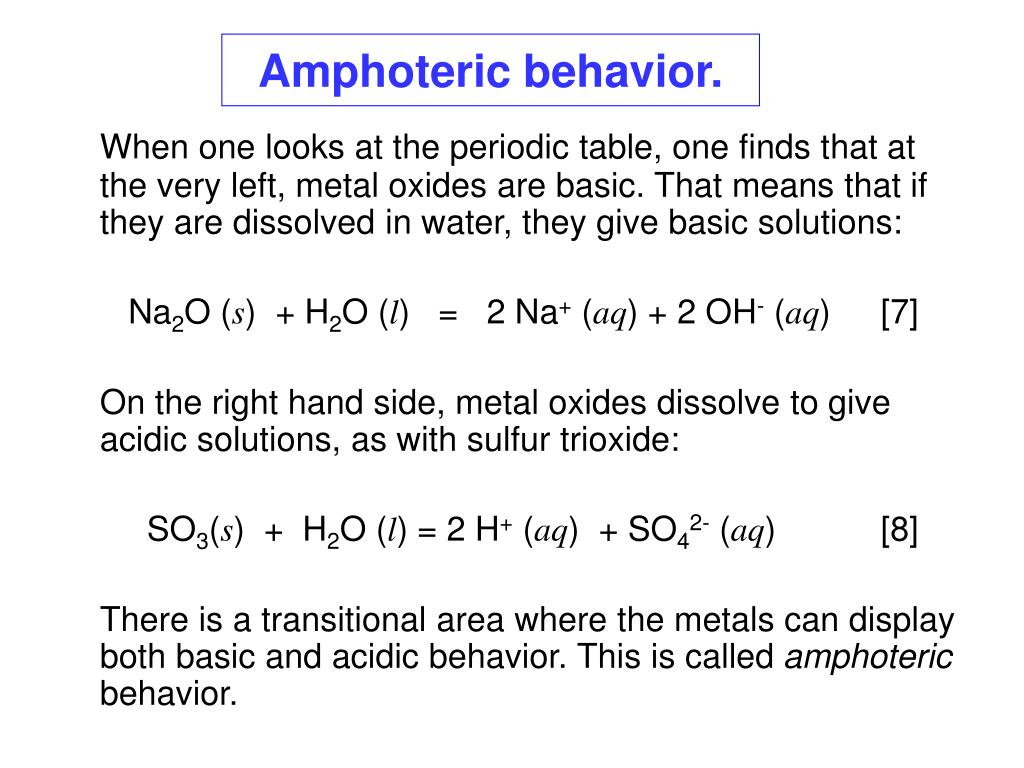
Where in the periodic table would you expect most amphoteric oxides to be located (2 points)? Image showing periodicity of the chemical elements for atomic number in a periodic table cityscape style. It has since been extended with the discovery of new metals, with the table remaining extremely useful in the field of chemistry as well as other scientific fields. So3 is more acidic than so2.
Image showing periodicity of the chemical elements for atomic number in a periodic table cityscape style. Metal oxides which react with both acids as well as bases to produce salts and water are known as amphoteric oxides. Visualize trends, 3d orbitals, isotopes, and mix compounds. Free videos no video selling business subscribe to support charity.
Many metals form amphoteric oxides or hydroxides. Visualize trends, 3d orbitals, isotopes, and mix compounds. The following sciencestruck article will cover some information related to metalloids. It has since been extended with the discovery of new metals, with the table remaining extremely useful in the field of chemistry as well as other scientific fields.